Ceramics is the collective name for pottery and porcelain. Traditional ceramics, also known as ordinary ceramics, is to clay and other natural silicates as the main raw material for firing products, modern ceramics, also known as new ceramics, fine ceramics or special ceramics. Commonly used non-silicate chemical raw materials or synthetic raw materials, such as oxides (alumina, zirconium oxide, titanium oxide, etc.) and non-oxide (silicon nitride, boron carbide, etc.) manufacturing.
Ceramics have many advantages such as excellent insulation, corrosion resistance, high temperature resistance, high hardness, low density, radiation resistance, etc. They have been widely used in various fields of national economy.
Traditional ceramic products, including household ceramics, architectural and sanitary ceramics, industrial arts ceramics, chemical ceramics, electrical ceramics, etc., are of various types and with different properties. With the rise of high-tech industry, a variety of new special ceramics have also achieved greater development, ceramics have become increasingly excellent structural and functional materials. They have higher temperature resistance than traditional ceramics, mechanical properties, special electrical properties and excellent chemical resistance.
Ceramics are also used as high-tech coatings for, for example, engine cylinders. Ceramics were developed in China in 620 AD and were introduced to Europe by Marco Polo around 1300 AD. It was not until 1708 that the first porcelain was produced in Europe. Thermal analysis allows the characterization of ceramic raw materials and the simulation of ceramic sintering processes, making the ceramics industry a classic application area for thermal analysis techniques.
Test conditions.
Temperature range: RT…1200°C
Sample mass: 37.14mg
Temperature rise rate: 20K/min
Test crucible: Pt
Test atmosphere: Synthetic air, 70 ml/min
Test conclusion.
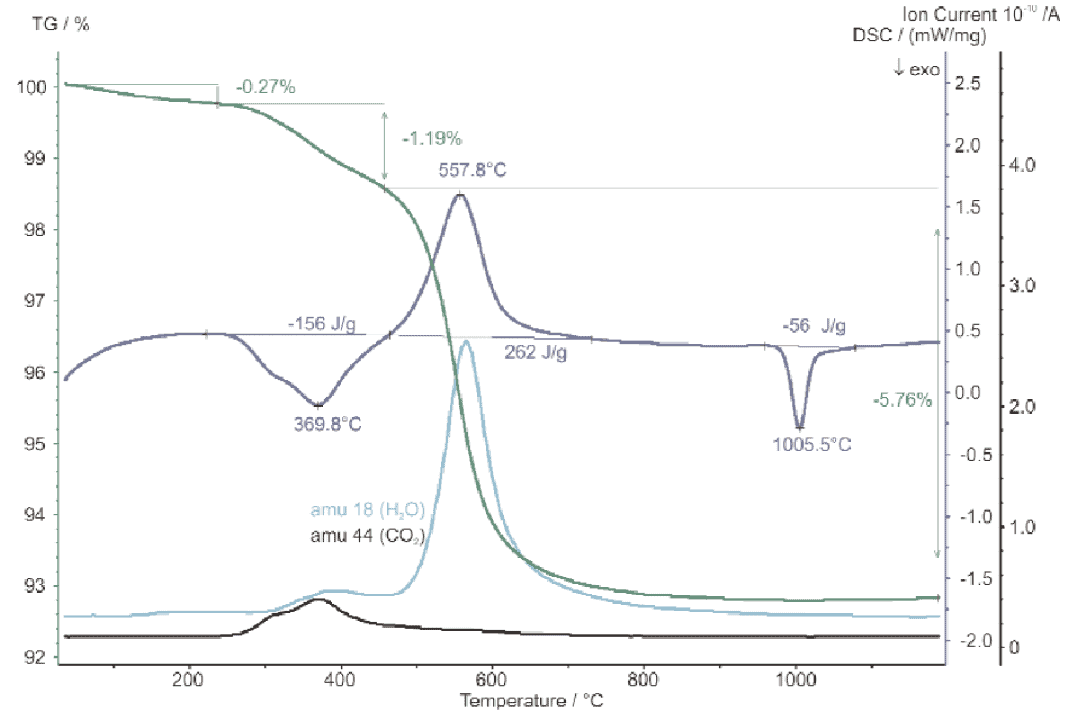
The STA test for ceramic raw materials showed three weight loss steps for the sample: weight loss up to 250°C, which is the volatilization of the sample water, and in the range from 250°C to 450°C, the loss of organic binder can be observed, accompanied by an exothermic heat of 156 J/g. Above 450°C, the weight loss is kaolin dehydration, which requires the absorption of 262 J/g. In the mass signal diagram, the mass numbers 18 and 44 The exothermic reaction at 1006°C is a solid phase transition of the kaolin component with an enthalpy of -56J/g. The reaction is reflected by the escape of H2O and CO2 gases.
Ceramic Parts India Inc. is a professional supplier of ceramic consumables for thermal analysis, please feel free to contact us if you need thermal analysis consumables for ceramic materials.